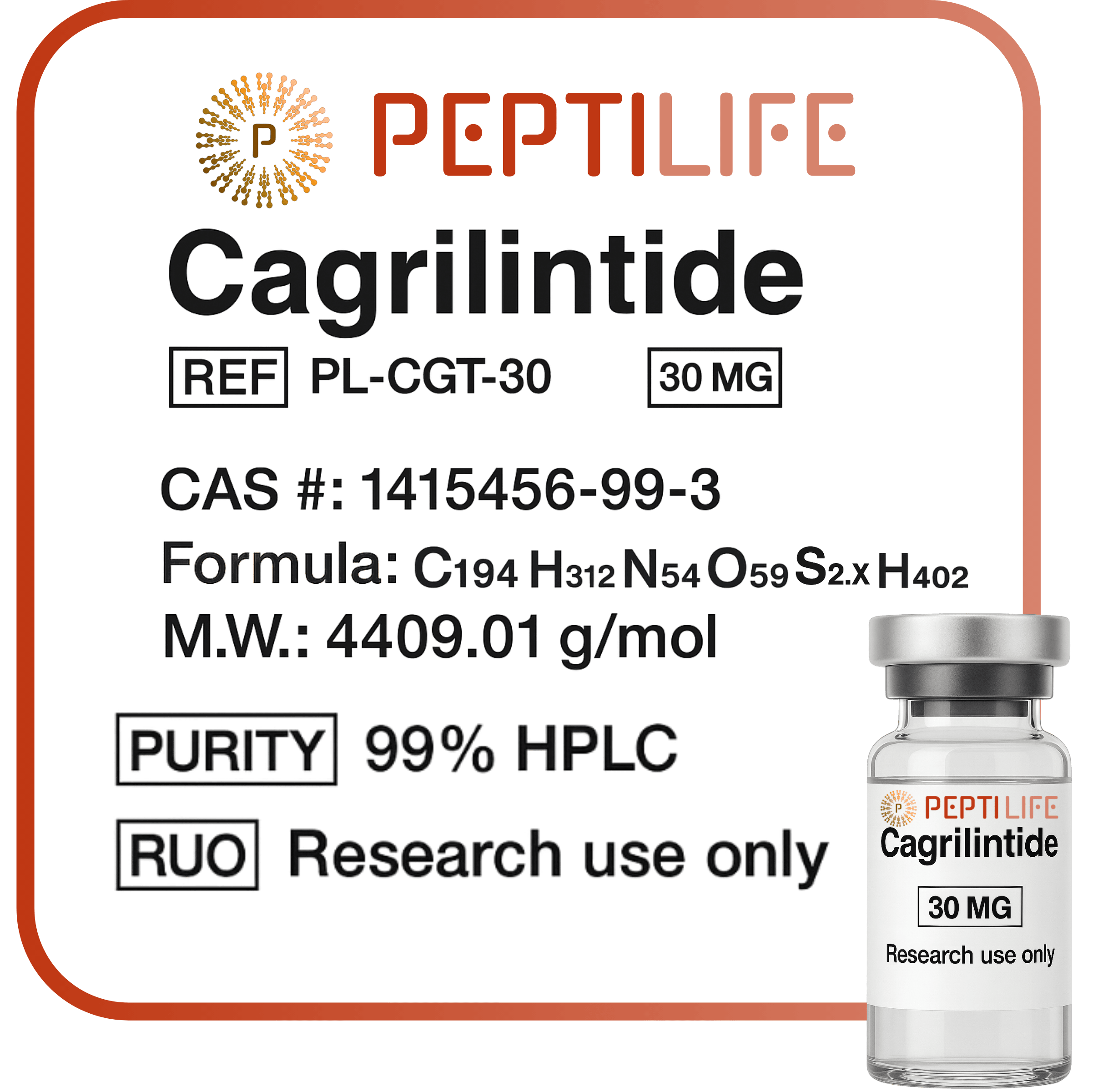
Cagrilintide 30mg
Next-Generation Amylin Analogue for Appetite Control and Sustained Satiety.
Formula: C₁₈₉H₂₉₁N₅₁O₅₉S (Molecular Weight ~ 4080 g/mol)
Description
Cagrilintide 30 mg is a long-acting synthetic amylin analogue designed to resist enzymatic degradation and sustain activity—offering powerful appetite suppression, delayed gastric emptying, and prolonged satiety through stable weekly dosing.
Mechanism of Action
Delays gastric emptying and promotes prolonged satiety;
Diminishes food cravings and reduces caloric intake;
Modulates appetite-regulating centers in the hypothalamus.
Clinical Applications
Primary or adjunctive treatment for obesity, including in non-diabetic patients;
Combination therapy notably with GLP-1 receptor agonists for enhanced weight loss efficacy;
Overweight or metabolically compromised patients requiring appetite regulation and weight control.
Clinical Data
In combination with semaglutide (as CagriSema), phase II/III trials demonstrated average weight loss of 15–20%, significantly outperforming monotherapies.
The REDEFINE program confirmed weight reductions of up to 20.4% over 68 weeks with favorable tolerability.
Cagrilintide 30 mg is provided as a lyophilized peptide (freeze-dried crystalline powder) to maximize stability. It remains stable for 3–4 months at room temperature and up to several years at –80°C. After reconstitution with bacteriostatic water, cagrilintide must be refrigerated at 2°C–8°C and used within 30 days.
Lyophilization (cryodesiccation) removes water under vacuum, producing a highly stable, crystalline peptide powder that resists hydrolysis. This process guarantees safe shipping worldwide without refrigeration, while preserving full biological activity until use.
Cagrilintide 30 mg is a long-acting amylin analogue peptide with the sequence XKCNTATCATQRLAEFLRHSSNNFGPILPPTNVGSNTP, molecular formula C₁₉₄H₃₁₂N₅₄O₅₉S₂C₂H₄O₂, and molecular weight of ~4409 g/mol. It is registered under CAS 1415456-99-3 and PubChem SID 171397054, with synonyms AT42613 and AM833.
The modifications to the amylin backbone confer resistance to enzymatic degradation, particularly by neprilysin, thereby extending half-life and enabling once-weekly dosing.
Mechanisms of action include:
Delaying gastric emptying;
Acting on hypothalamic satiety centers;
Reducing caloric intake and suppressing cravings;
Complementing GLP-1 analogues in synergistic weight management.
Origin: Amylin (also called islet amyloid polypeptide, IAPP) is a natural 37-amino acid hormone co-secreted with insulin. It regulates satiety, slows glucose absorption, and modulates energy metabolism. Cagrilintide, as a modified analogue, enhances these properties with greater potency and durability.
Cagrilintide 30 mg is synthesized and processed under GMP-certified environments, ensuring the highest pharmaceutical-grade standards. Lyophilization under aseptic conditions guarantees long-term stability. Each production batch is validated by HPLC, peptide mapping, and MS analysis, confirming sequence integrity and >99% purity.
Excipients such as sodium phosphate buffer, trehalose, and polysorbate 80 are incorporated to maintain solubility, prevent aggregation, and stabilize the peptide during reconstitution. These excipients are pharmacopeial grade, tested for sterility and biocompatibility.
Rigorous endotoxin testing ensures bacterial contamination is far below accepted pharmacopeial limits. Each vial is sealed for sterility assurance and individually labeled for traceability, with lot number and expiration date clearly displayed.
Cagrilintide 30 mg is a long-acting amylin analogue, structurally optimized to resist enzymatic degradation by neprilysin, thereby extending half-life and maintaining therapeutic activity for up to one week per dose.
Pharmacodynamically, Cagrilintide:
Delays gastric emptying to prolong satiety;
Acts on hypothalamic satiety centers to reduce food cravings;
Decreases caloric intake and body weight;
Improves metabolic flexibility when combined with GLP-1 receptor agonists.
Clinical evidence shows that in CagriSema (Cagrilintide + Semaglutide) trials, patients achieved up to 20% body weight reduction over 68 weeks, outperforming either monotherapy. This highlights its role not only as a monotherapy candidate but also as a synergistic partner in combination regimens.
The safety profile is favorable, with gastrointestinal side effects being the most common, usually transient. No clinically significant cardiovascular or hepatic adverse events have been reported to date in ongoing trials.
Where science meets vitality.
Your journey to a better you starts here.
© 2025. All rights reserved.


